The patentability of antibodies in Brazil
It is concluded that the BPTO, EPO and USPTO have different understandings about this matter and about how to claim antibodies. In this context, the BPTO takes a stricter position when compared to other Offices.
segunda-feira, 3 de outubro de 2022
Atualizado às 10:41
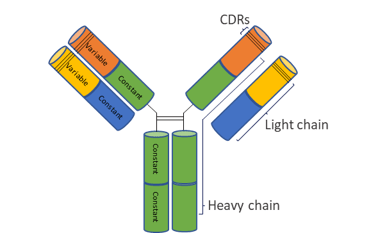
Antibodies, also called immunoglobulins (Ig), are proteins that act in the defense of organisms binding to “foreign bodies”, the antigens. Most immunoglobulins are formed by a combination of light and heavy chains arranged in a shape similar to the letter Y.
The light and heavy chains are formed by constant and variable regions, the latter being responsible for the formation of antigen binding sites and, consequently, for the specificity of antibodies. Within each variable region, there are three small regions that create the antigen-binding surface, called the complementarity-determining regions (CDRs).
Brazilian law and practices
Based on the provisions of article 10(IX)1 of the Brazilian IP Law (Law 9,279/96), it can be said that antibodies, whether engineered or not, that have a natural counterpart or are obtained from an organism naturally exposed to the antigen will not be considered an invention.
In addition, according to the Brazilian PTO (BPTO), natural biological products, even if isolated from nature - that is, extracted from their natural context and subjected to an isolation or purification process - are also not considered an invention (Normative Instruction NI/BPTO/PR 118/202 , items 4.1 and 4.2.1.1).
As well as full-length molecules, fragments originated from antibodies found in nature or that are part of other natural proteins are also not privileged in terms of article 10(IX) of the Brazilian IP Law.
On the other hand, antibodies produced from human intervention are patentable, either by repetitive and controlled exposure to an antigen using adjuvants, by production from hybridomas or by their molecular engineering.
Particularly, when antibodies are the main object of the invention, they must be defined by their linear amino acid sequences, contained in the sequence listing and identified by their SEQ ID Nos or by means of the deposit number of the corresponding hybridoma in an International Depositary Authority (IDA) of one of the member countries of the Budapest Treaty, provided that such deposit has been made prior to the filing date of the patent application or the filing date of the priority application, when applicable.
The BPTO does not accept antibodies being characterized only by their properties, such as their function of binding to a certain target or their physicochemical characteristics. Still, it is necessary to minimally define the sequences of the three CDRs of each chain present so that the antibody is defined clearly and precisely, in line with the provisions of article 253 of the Brazilian IP Law and Normative Instruction NI/BPTO/PR 118/20 (item 6.1 and Example 36).
It should be noted that the definition of biological sequences by percentage of identity is also not accepted by the BPTO. In addition to lacking clarity and precision, it is understood that such a definition is very broad and encompasses, in its scope of protection, sequences not supported by the specification or that do not meet the patentability requirements of novelty and inventive activity (Normative Instruction NI/BPTO/PR 118/20, item 6.2).
European Patent Office (EPO) and US Patent and Trademark Office (USPTO)
Unlike the BPTO, both the EPO and the USPTO accept, in principle, antibodies being defined by their functions and their biological sequences being defined by percentage of identity.
For European patent applications, if the percentage identity is the only characteristic to define the antibody, it is necessary to clearly determine the percentage identity calculation in the specification (Guidelines for Examination in the European Patent Office4 - F-IV, 4.24).
In the US patent applications, for a genus claim to meet the requirements of 35 U.S.C. § 112(a)5, for example, when claiming antibodies by percentage of identity, it is highlighted that it is necessary to show several examples of sequences within the range of percentage of interest and/or to discuss the substitutions that can be made without the antibody function being compromised (2163 Guidelines for the Examination of Patent Applications Under the 35 U.S.C. 112(a) or Pre-AIA 35 U.S.C. 112, first paragraph, "Written Description" Requirement [R-10.2019]6 – item 3.II.ii7).
Thus, it is concluded that the BPTO, EPO and USPTO have different understandings about this matter and about how to claim antibodies. In this context, the BPTO takes a stricter position when compared to other Offices.
Recommendations
Based on the above, Applicants seeking protection in Brazil for inventions in this technical field should adapt the patent applications in accordance with the provisions of the Brazilian IP Law (Law 9.279/96) and NI/BPTO/PR 118/20 before the filling.
Particularly, it would be advisable to expressly include information about of:
- at least the hybridoma deposit number in their specification; or
- the linear amino acid sequences corresponding to the CDRs in the sequence listing in the patent application.
______________
1 Art. 10 - The following are not considered to be inventions or utility models: (…)
IX. the whole or part of natural living beings and biological materials found in nature, even if isolated therefrom, including the genome or germ plasm from any natural living being, and the natural biological processes.
2 Normative Instruction NI/BPTO/PR nº 118/2020.
3 Article 25 - The claims must be based on the specification, characterizing the particularities of the application, clearly and precisely defining the subject matter that is the object of the protection.
4 Guidelines for Examination in the European Patent Office – F-IV, 4.24.
5 The specification shall contain a written description of the invention, and of the manner and process of making and using it, in such full, clear, concise, and exact terms as to enable any person skilled in the art to which it pertains, or with which it is most nearly connected, to make and use the same, and shall set forth the best mode contemplated by the inventor or joint inventor of carrying out the invention.
6 2163 Guidelines for the Examination of Patent Applications Under the 35 U.S.C. 112(a) or Pre-AIA 35 U.S.C. 112, first paragraph, “Written description” Requirement [R-10.2019].
7 The written description requirement for a claimed genus may be satisfied through sufficient description of a representative number of species by actual reduction to practice, reduction to drawings, or by disclosure of relevant, identifying characteristics, i.e., structure or other physical and/or chemical properties, by functional characteristics coupled with a known or disclosed correlation between function and structure, or by a combination of such identifying characteristics, sufficient to show the applicant was in possession of the claimed genus. See Eli Lilly, 119 F.3d at 1568, 43 USPQ2d at 1406.
Bianca Bassetto Bissoni
Graduada em Biotecnologia pela Universidade Federal de São Carlos (UFSCAR), São Paulo, 2018.


